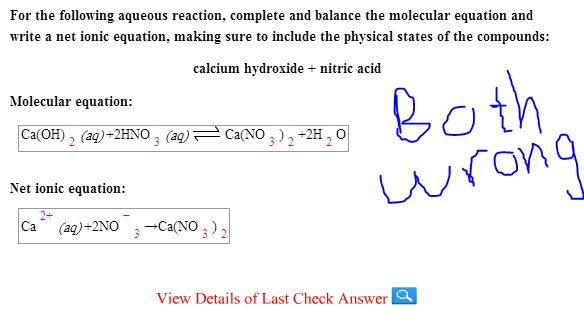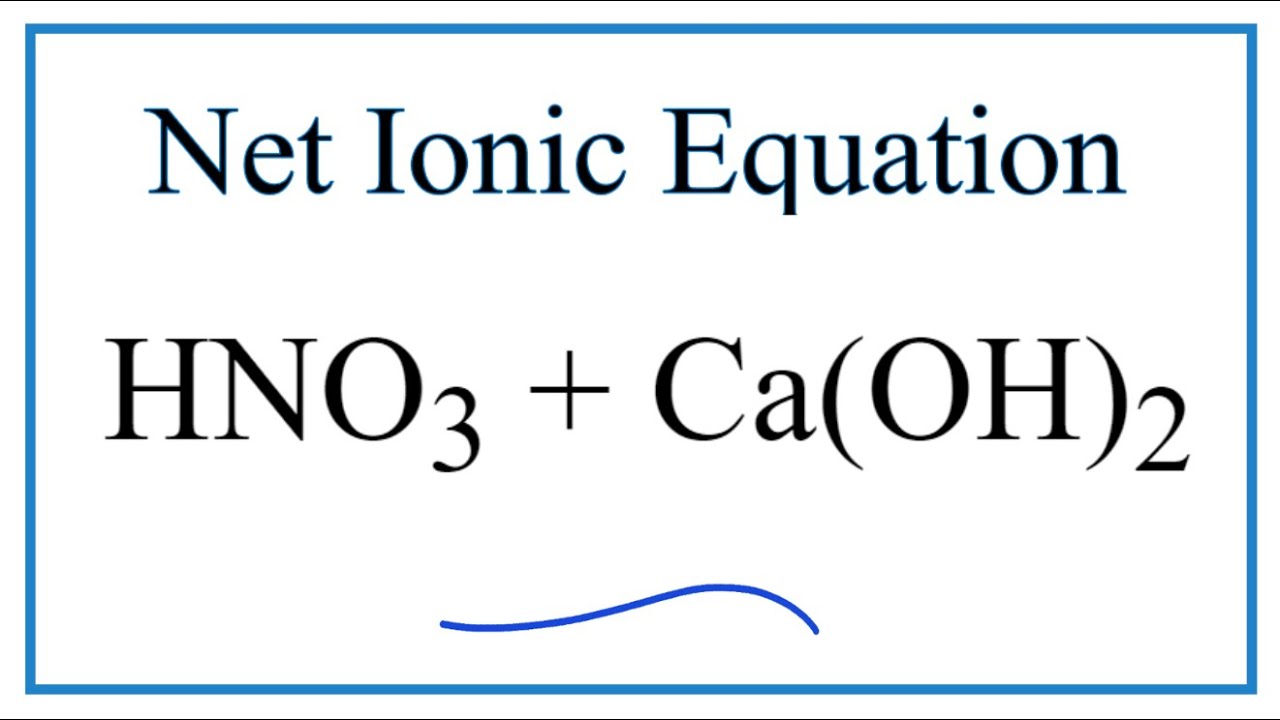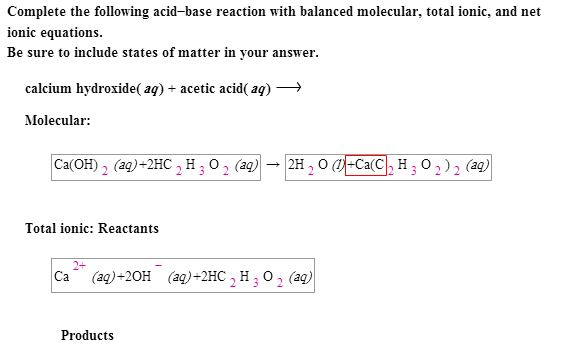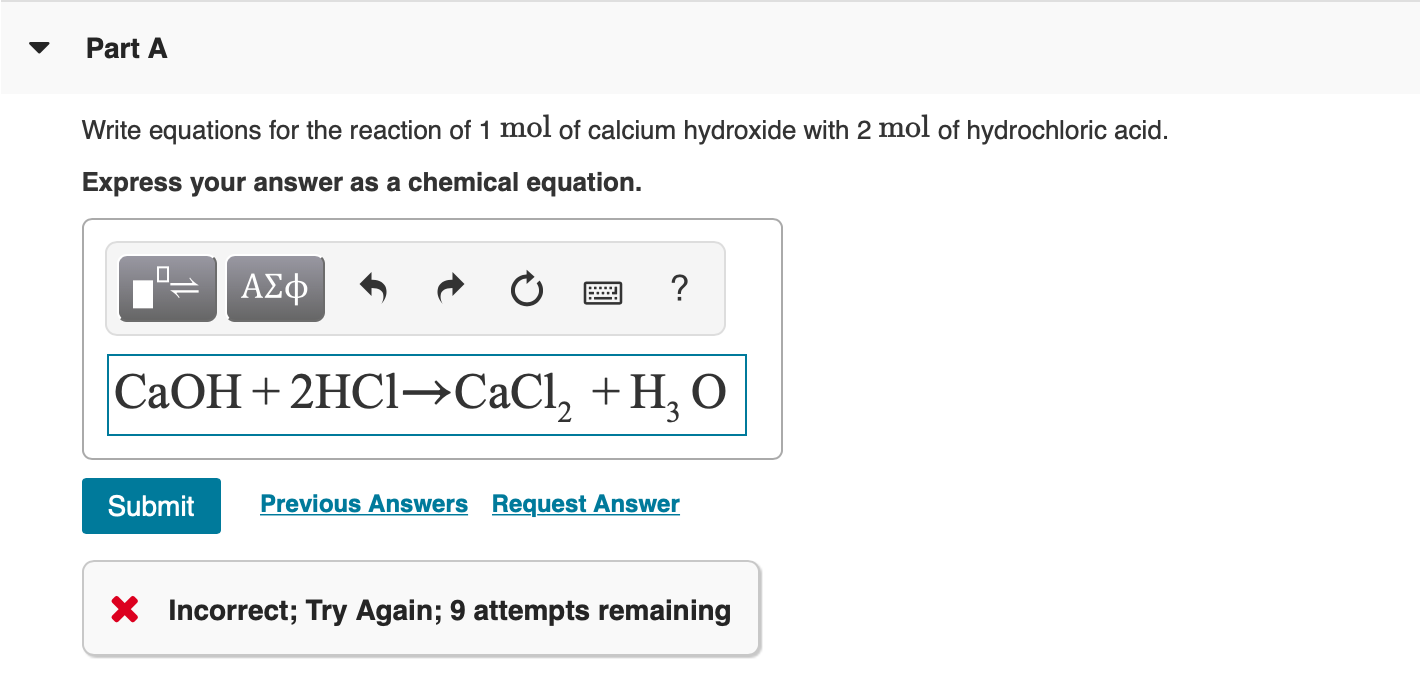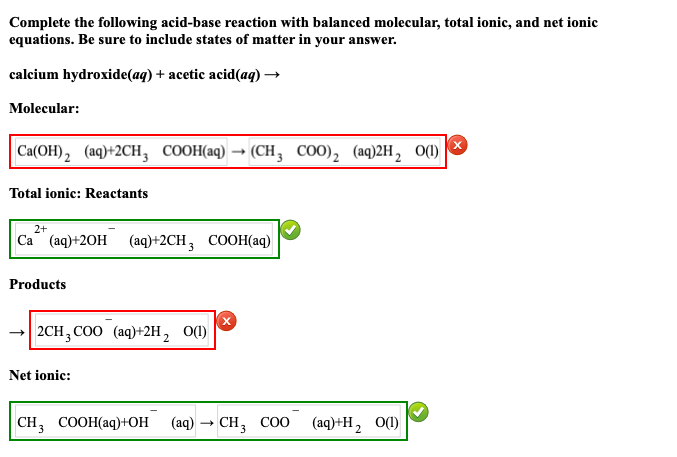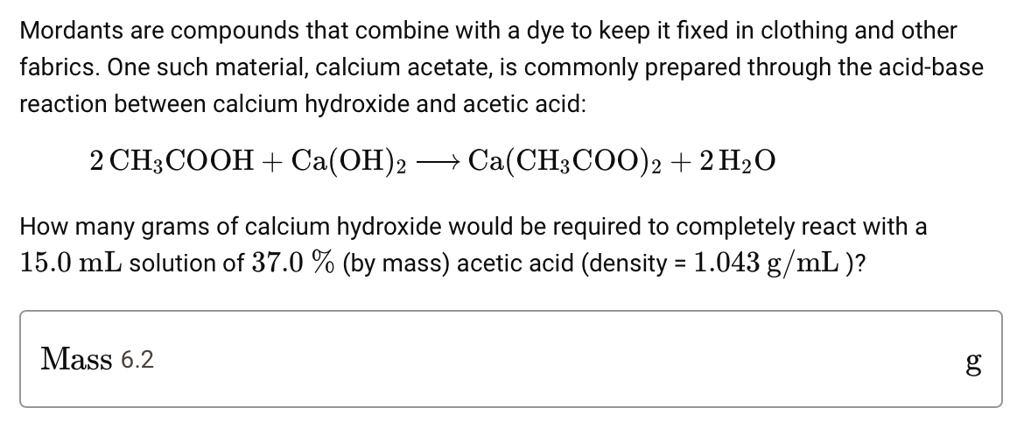
SOLVED: Mordants are compounds that combine with a dye to keep it fixed in clothing and other fabrics. One such material, calcium acetate, is commonly prepared through the acid-base reaction between calcium
![Calcium hydroxide is a strong base. Compute `[Ca^(2+)]` and `[OH^(-)]` for a solution that is pr... - YouTube Calcium hydroxide is a strong base. Compute `[Ca^(2+)]` and `[OH^(-)]` for a solution that is pr... - YouTube](https://i.ytimg.com/vi/wtPIrhTYyLE/maxresdefault.jpg)
Calcium hydroxide is a strong base. Compute `[Ca^(2+)]` and `[OH^(-)]` for a solution that is pr... - YouTube

Introduction to Acids and Bases. Acid A substance that produces hydrogen ions, H + (aq), when it dissolves in water. Sour-tasting and good conductors. - ppt download

ACIDS & BASES module i.An acid is a chemical substance that …………………in water to produce ………………. ions. ii.A base is a chemical substance that ………………in. - ppt download